Goldman Sachs has begun coverage of Alkermes (ALKS, Financial) with a positive outlook, assigning a Buy rating and setting a price target of $43. The firm points to the company's established portfolio in the neuropsychological field as being well-positioned to meet its fiscal 2025 objectives. Additionally, recent developments in Alkermes' orexin receptor 2 portfolio are expected to contribute to rising share prices. Goldman Sachs views the upcoming data releases as catalysts for further stock appreciation.
Wall Street Analysts Forecast
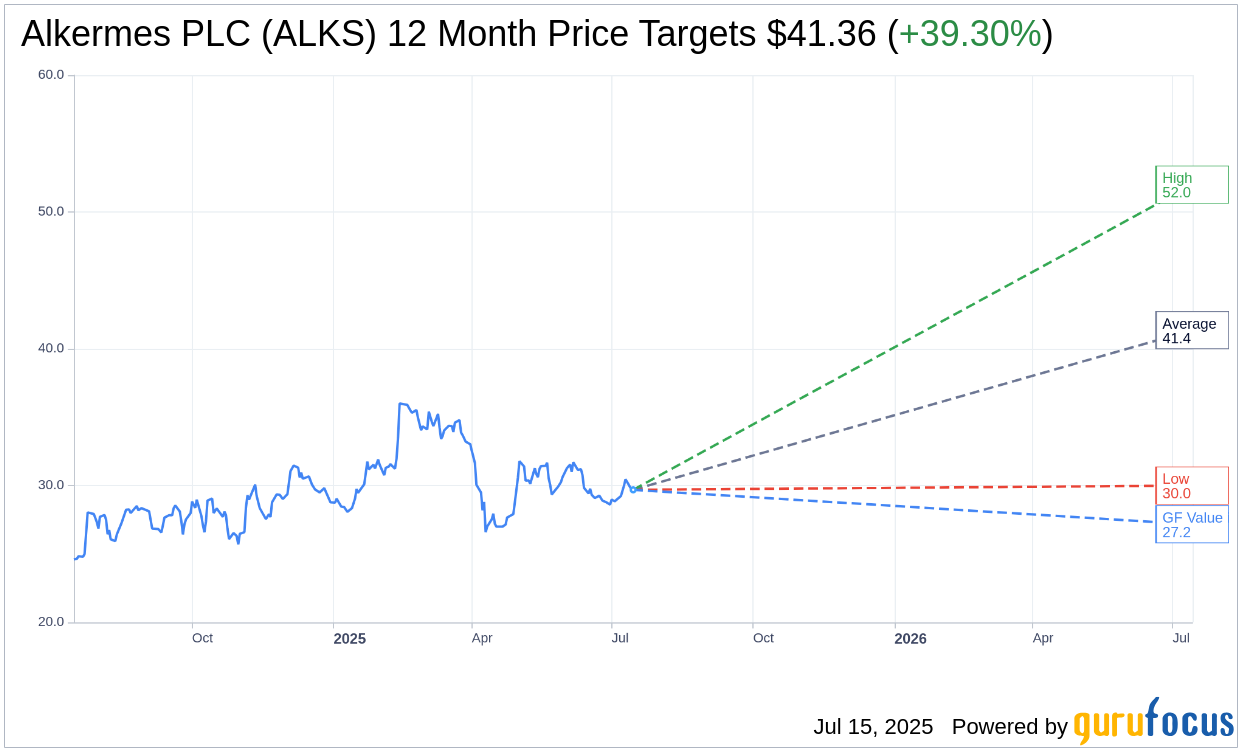
Based on the one-year price targets offered by 14 analysts, the average target price for Alkermes PLC (ALKS, Financial) is $41.36 with a high estimate of $52.00 and a low estimate of $30.00. The average target implies an upside of 39.30% from the current price of $29.69. More detailed estimate data can be found on the Alkermes PLC (ALKS) Forecast page.
Based on the consensus recommendation from 16 brokerage firms, Alkermes PLC's (ALKS, Financial) average brokerage recommendation is currently 2.1, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
Based on GuruFocus estimates, the estimated GF Value for Alkermes PLC (ALKS, Financial) in one year is $27.17, suggesting a downside of 8.49% from the current price of $29.69. GF Value is GuruFocus' estimate of the fair value that the stock should be traded at. It is calculated based on the historical multiples the stock has traded at previously, as well as past business growth and the future estimates of the business' performance. More detailed data can be found on the Alkermes PLC (ALKS) Summary page.
ALKS Key Business Developments
Release Date: May 01, 2025
- Total Revenue: $306.5 million for Q1 2025.
- Net Sales from Proprietary Products: $244.5 million, reflecting 5% year-over-year growth.
- Vivitrol Net Sales: $101 million for Q1 2025.
- Aristada Net Sales: $73.5 million for Q1 2025.
- Lybalvi Net Sales: $70 million, with 23% year-over-year growth.
- Manufacturing and Royalty Revenues: $62 million for Q1 2025.
- Cost of Goods Sold: $49.2 million, down from $58.6 million in Q1 last year.
- R&D Expenses: $71.8 million, up from $67.6 million in Q1 last year.
- SG&A Expenses: $171.7 million, down from $179.7 million in Q1 last year.
- GAAP Net Income: $22.5 million for Q1 2025.
- EBITDA: $22.8 million for Q1 2025.
- Adjusted EBITDA: $45.6 million for Q1 2025.
- Cash and Investments: $916.2 million at the end of Q1 2025.
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- Alkermes PLC (ALKS, Financial) reported first-quarter net sales of $244.5 million from its proprietary product portfolio, slightly above expectations, driven by a 5% year-over-year growth.
- The company is making significant progress in its ALKS 2680 program, with the NT1 study, Vibrance One, fully enrolled and top-line results expected early in the third quarter.
- Alkermes PLC (ALKS) maintains a strong financial position with over $900 million in cash and investments, providing flexibility to adapt to macroeconomic changes.
- The company has completed the expansion of its psychiatry sales force, which is expected to drive growth for its products, particularly Lybalvi and Aristada.
- Alkermes PLC (ALKS) is strategically positioned with US-based manufacturing, minimizing potential impacts from tariffs and foreign reference pricing.
Negative Points
- The passing of a key financial officer, Ian Brown, has left a gap in leadership, with Blair Jackson serving as interim principal financial officer.
- The company faces potential challenges from Medicaid changes and FDA regulations, which could impact its business operations.
- Alkermes PLC (ALKS) anticipates increased R&D expenses due to ongoing phase two studies, which could affect profitability.
- There are concerns about potential visual disturbances in the ALKS 2680 program, although the company has not observed significant issues so far.
- The company is preparing for potential generic competition for Vivitrol in the coming years, which could impact future revenue.
