Nexalin Technology, with ticker symbol NXL, is preparing to initiate a Q-Submission with the U.S. Food and Drug Administration. This move aims to facilitate detailed discussions with the agency about the design of clinical trials for its Gen-2 SYNC system. The focus is on conditions like Alzheimer's disease, dementia, and mild cognitive impairment. The decision to proceed follows the release of recent studies, an analysis of promising internal data, and early insights from the FDA. Nexalin views this regulatory engagement as a vital step in advancing Gen-2 SYNC as a non-invasive treatment option for individuals struggling with severe cognitive disorders.
Wall Street Analysts Forecast
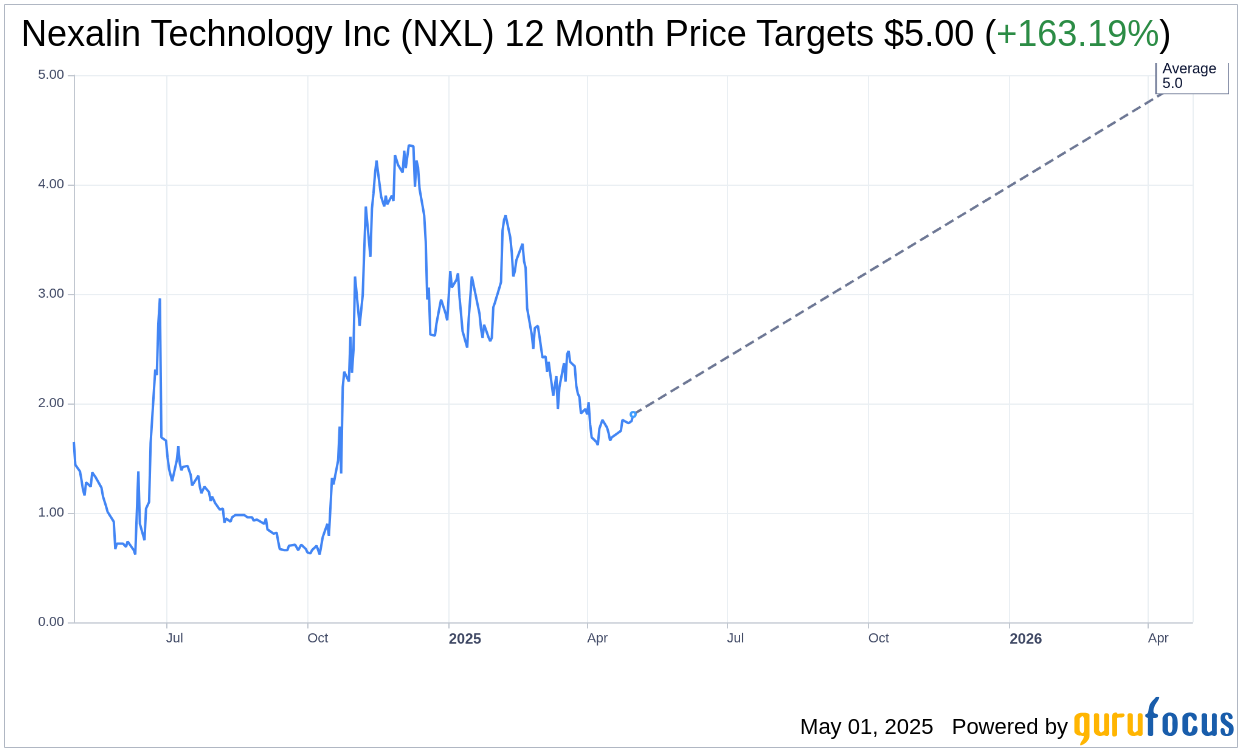
Based on the one-year price targets offered by 1 analysts, the average target price for Nexalin Technology Inc (NXL, Financial) is $5.00 with a high estimate of $5.00 and a low estimate of $5.00. The average target implies an upside of 163.19% from the current price of $1.90. More detailed estimate data can be found on the Nexalin Technology Inc (NXL) Forecast page.
Based on the consensus recommendation from 1 brokerage firms, Nexalin Technology Inc's (NXL, Financial) average brokerage recommendation is currently 2.0, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.