In the first quarter, Genmab (GMAB, Financial) showcased its dedication to advancing its late-stage programs. The company successfully secured additional territory approvals for both EPKINLY and Tivdak. Furthermore, the updated data from Rina-S, presented at the Society of Gynecologic Oncology meeting, highlighted its potential as a treatment for advanced ovarian cancer.
Wall Street Analysts Forecast
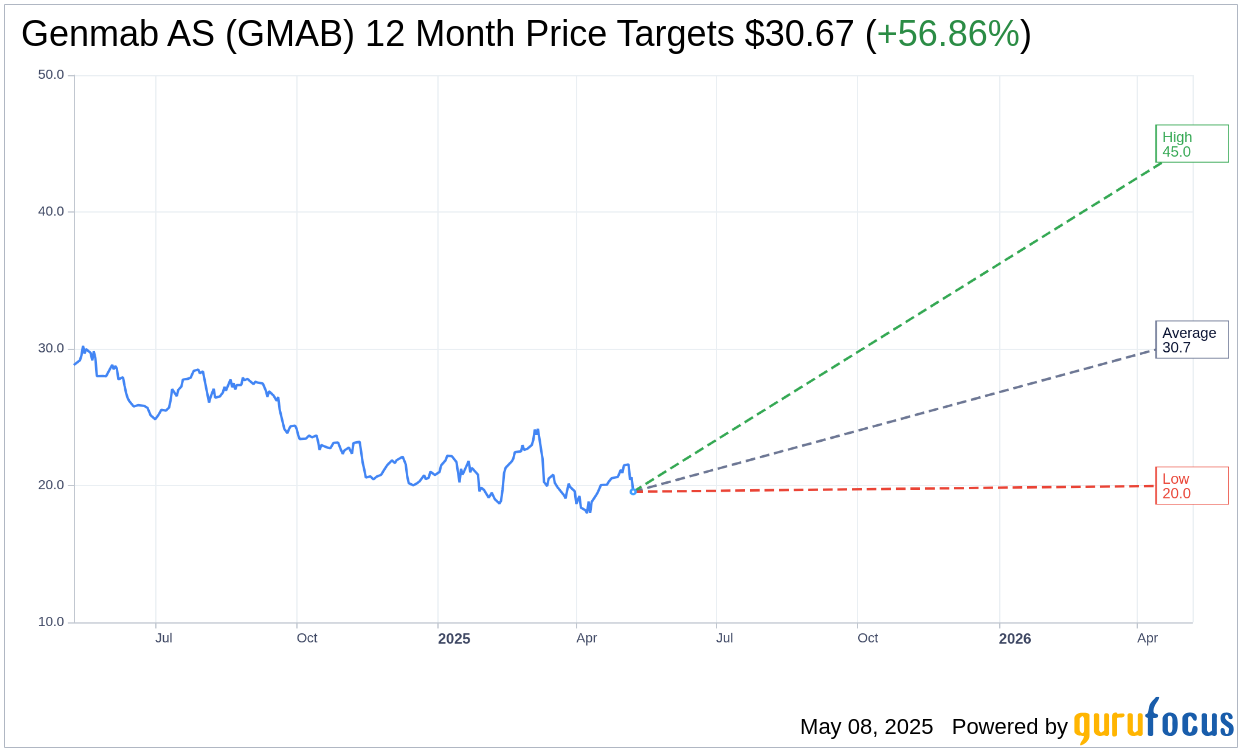
Based on the one-year price targets offered by 9 analysts, the average target price for Genmab AS (GMAB, Financial) is $30.67 with a high estimate of $45.00 and a low estimate of $20.00. The average target implies an upside of 56.86% from the current price of $19.55. More detailed estimate data can be found on the Genmab AS (GMAB) Forecast page.
Based on the consensus recommendation from 12 brokerage firms, Genmab AS's (GMAB, Financial) average brokerage recommendation is currently 2.0, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
Based on GuruFocus estimates, the estimated GF Value for Genmab AS (GMAB, Financial) in one year is $64.92, suggesting a upside of 232.07% from the current price of $19.55. GF Value is GuruFocus' estimate of the fair value that the stock should be traded at. It is calculated based on the historical multiples the stock has traded at previously, as well as past business growth and the future estimates of the business' performance. More detailed data can be found on the Genmab AS (GMAB) Summary page.
GMAB Key Business Developments
Release Date: February 12, 2025
- Total Revenue Growth: 31% increase in 2024.
- Operating Profit Growth: 26% increase in 2024.
- Cash Position: Nearly $3 billion at year-end 2024.
- Acquisition: $1.8 billion acquisition of ProfoundBio.
- Share Buyback: $500 million in 2024.
- EPKINLY Sales: $281 million in 2024.
- Tivdak Sales: $131 million in 2024.
- Recurring Revenue Growth: 35% increase in 2024.
- DARZALEX Net Sales: Nearly $11.7 billion in 2024.
- Operating Expenses: DKK13.8 billion in 2024.
- Effective Tax Rate: 14.4% in 2024.
- Net Profit: Nearly DKK7.8 billion in 2024.
- 2025 Revenue Guidance: $3.3 billion to $3.7 billion.
- 2025 Operating Profit Guidance: $895 million to $1.4 billion.
- 2025 Recurring Revenue Growth: Expected 18% increase.
- 2025 DARZALEX Sales Projection: $12.6 billion to $13.4 billion.
- Share Repurchase Plan: Approximately $370 million in 2025.
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- Genmab AS (GMAB, Financial) achieved a 31% total revenue growth in 2024, driven by the success of its eight commercialized medicines, including EPKINLY and Tivdak.
- The company ended the year with nearly $3 billion in cash, reinforcing its financial strength and flexibility for future investments.
- Genmab AS (GMAB) has 12 products or product candidates in 30 clinical trials, including 7 Phase III trials, indicating a robust pipeline.
- EPKINLY has received multiple regulatory approvals and is expected to achieve peak sales exceeding $3 billion, highlighting its market potential.
- The acquisition of ProfoundBio and the integration of Rina-S into the pipeline have been successful, with plans for two Phase III trials underway, showcasing effective strategic investments.
Negative Points
- Despite strong financial performance, the company faces significant competition in the B-cell malignancies market, which could impact EPKINLY's market share.
- The $1.8 billion acquisition of ProfoundBio and a $500 million share buyback represent substantial financial commitments that could strain resources if expected returns are not realized.
- The company's guidance for 2025 includes a decrease in nonrecurring revenue by more than $100 million, which could affect overall revenue growth.
- There is uncertainty regarding the timing and outcome of regulatory approvals for key products like Tivdak in Europe, which could delay market expansion.
- The development of new indications for products like acasunlimab and Rina-S involves high-risk clinical trials, which may not yield successful outcomes.
