Piper Sandler has revised its price target for InspireMD (NSPR, Financial), lowering it from $4.50 to $4, while maintaining an Overweight rating on the stock. This adjustment follows the company's first-quarter results, which surpassed sales expectations. Despite the strong sales performance, InspireMD has adjusted its forecast for FDA approval of the CGuard Prime stent, now expected in the third quarter of 2025, instead of the earlier timeline of the first half of 2025. This change is attributed to factors outside the company's control. Additionally, the anticipated U.S. launch of the SwitchGuard TCAR system has shifted slightly, with expectations now set for late 2026.
Wall Street Analysts Forecast
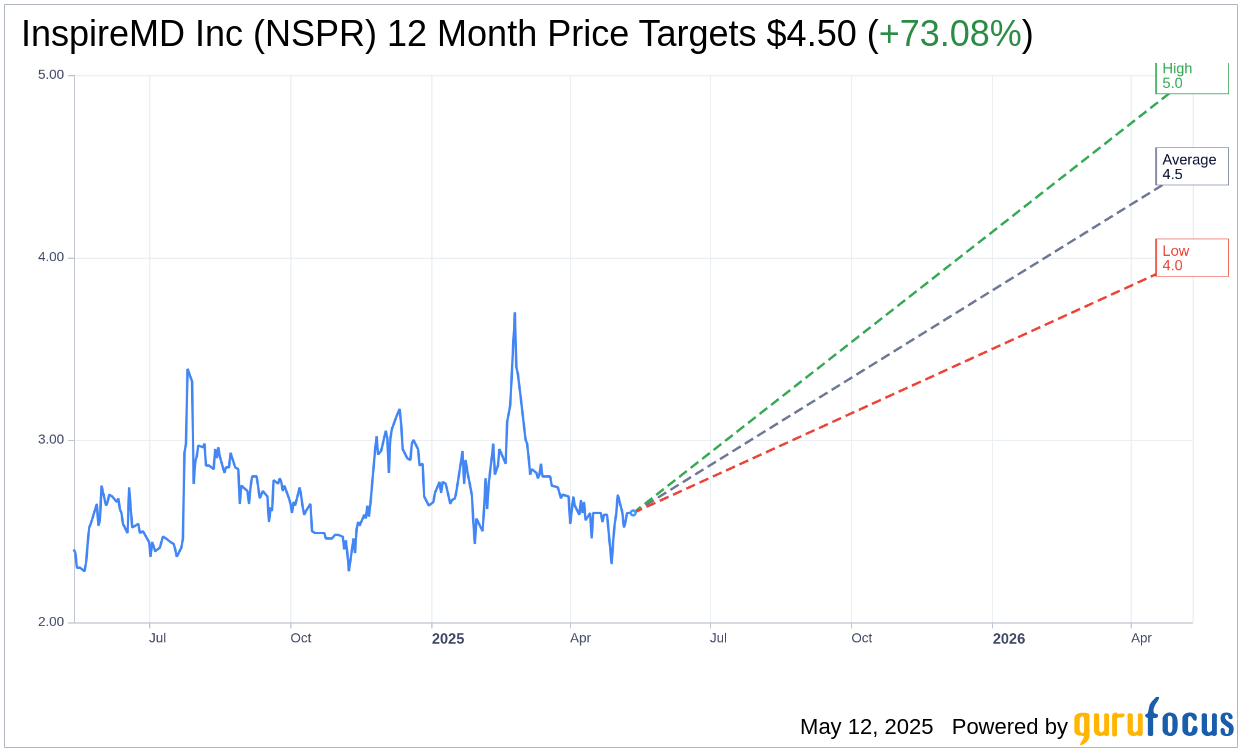
Based on the one-year price targets offered by 2 analysts, the average target price for InspireMD Inc (NSPR, Financial) is $4.50 with a high estimate of $5.00 and a low estimate of $4.00. The average target implies an upside of 73.08% from the current price of $2.60. More detailed estimate data can be found on the InspireMD Inc (NSPR) Forecast page.
Based on the consensus recommendation from 2 brokerage firms, InspireMD Inc's (NSPR, Financial) average brokerage recommendation is currently 2.0, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
Based on GuruFocus estimates, the estimated GF Value for InspireMD Inc (NSPR, Financial) in one year is $0.69, suggesting a downside of 73.46% from the current price of $2.6. GF Value is GuruFocus' estimate of the fair value that the stock should be traded at. It is calculated based on the historical multiples the stock has traded at previously, as well as past business growth and the future estimates of the business' performance. More detailed data can be found on the InspireMD Inc (NSPR) Summary page.
NSPR Key Business Developments
Release Date: May 09, 2025
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- InspireMD Inc (NSPR, Financial) reported a 1.2% year-over-year growth in revenue, reaching $1.53 million for the first quarter of 2025.
- The company has sold approximately 64,000 Seaguard stents to date, showcasing its expertise and strong market presence.
- InspireMD Inc (NSPR) is optimistic about receiving FDA approval for Seaguard Prime in the third quarter of 2025, which is expected to drive significant demand.
- The company has built a strong commercial and operational team in anticipation of the US launch, with 20 high-powered sales and marketing professionals already onboarded.
- InspireMD Inc (NSPR) is advancing its clinical pipeline with strong enrollment in the Sea Guardians 2 pivotal study and plans for the Sea Guardians 3 IDE submission.
Negative Points
- The company's gross profit for the first quarter of 2025 remained flat compared to the previous year, with a gross margin of only 19.1%.
- Operating expenses increased by 52.5% to $11.75 million, primarily due to higher salaries and launch preparations.
- Net loss for the first quarter of 2025 was $11.17 million, a significant increase from the previous year's net loss of $732,000.
- Cash and cash equivalents decreased substantially to $2.69 million as of March 30, 2025, from $34.64 million at the end of 2024.
- The timeline for FDA approval of Seaguard Prime has been slightly pushed to Q3 2025 due to delays in the facility site audit and subsequent feedback process.