Lexicon Pharmaceuticals (LXRX, Financial) reported its first-quarter revenue at $1.26 million, surpassing the market expectation of $1.18 million. The company achieved significant milestones early in 2025, including securing an exclusive licensing deal with Novo Nordisk for their compound LX9851, targeting obesity and related conditions.
The company also shared promising updates from its PROGRESS Phase 2b study for pilavapadin in diabetic peripheral neuropathic pain (DPNP). They have identified a 10 mg dose to advance into Phase 3 trials and are preparing for their end of Phase 2 meeting with the FDA.
Looking ahead, Lexicon plans to enhance research on sotagliflozin to provide unique insights into heart failure treatment. They are also focused on progressing with the Phase 3 SONATA-HCM study, which seeks to validate the effectiveness and safety of sotagliflozin in treating both obstructive and non-obstructive hypertrophic cardiomyopathy. These efforts position the company to strengthen its pipeline and continue its growth trajectory in 2025.
Wall Street Analysts Forecast
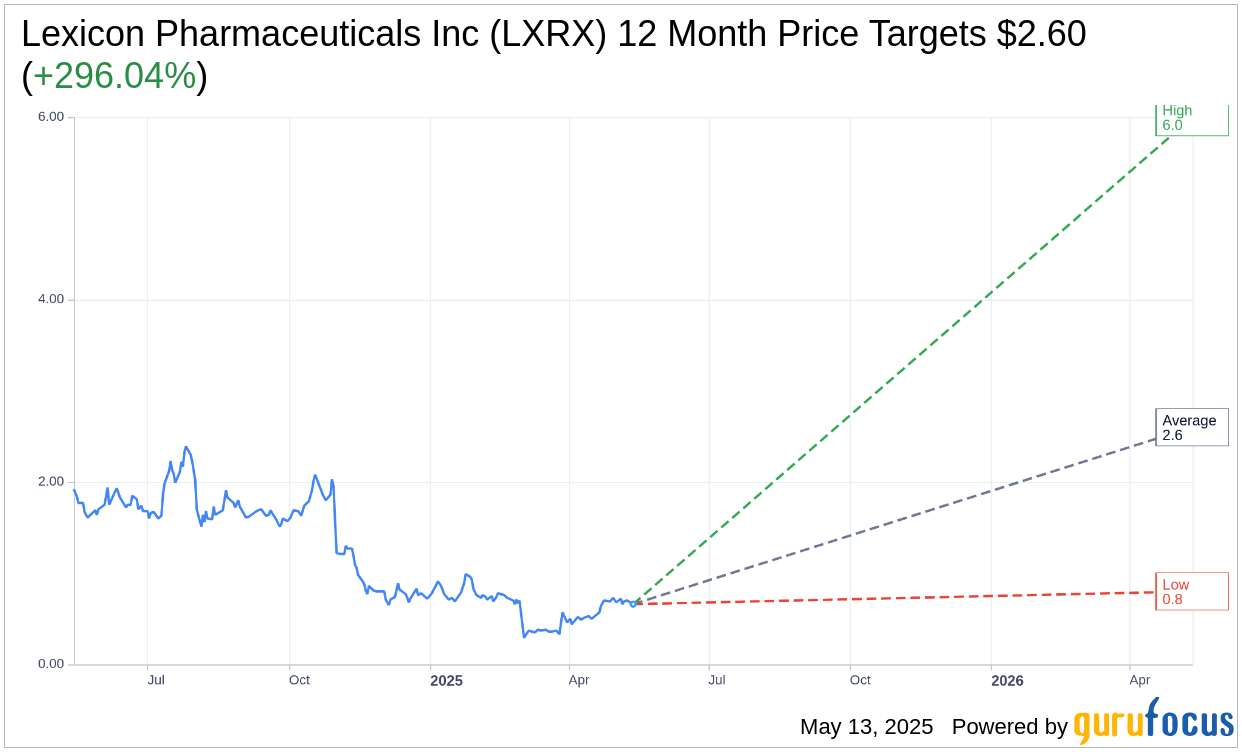
Based on the one-year price targets offered by 5 analysts, the average target price for Lexicon Pharmaceuticals Inc (LXRX, Financial) is $2.60 with a high estimate of $6.00 and a low estimate of $0.80. The average target implies an upside of 309.06% from the current price of $0.64. More detailed estimate data can be found on the Lexicon Pharmaceuticals Inc (LXRX) Forecast page.
Based on the consensus recommendation from 5 brokerage firms, Lexicon Pharmaceuticals Inc's (LXRX, Financial) average brokerage recommendation is currently 2.2, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
LXRX Key Business Developments
Release Date: March 06, 2025
- Cash Equivalents and Short-term Investments: $238 million as of December 31, 2024, compared to $170 million as of December 30, 2023.
- Revenue: $26.6 million in Q4 2024 and $31.1 million for the full year 2024.
- MEA Revenue: $1.7 million in Q4 2024 and $6 million for the full year 2024.
- Upfront Payment: $25 million received from the Vatrice licensing agreement.
- Research and Development Expenses: $26.7 million in Q4 2024, up from $14.8 million in Q4 2023; $84.5 million for the full year 2024.
- Selling, General and Administrative Expenses: $32.3 million in Q4 2024, comparable to $32.6 million in Q4 2023; $143.1 million for the full year 2024, up from $114 million in 2023.
- Net Loss: $33.8 million or $0.09 per share in Q4 2024, compared to $49.8 million or $0.20 per share in Q4 2023; $200.4 million or $0.32 per share for the full year 2024, compared to $177.1 million or $0.80 per share in 2023.
- 2025 Operating Expenses Guidance: Expected to be in the range of $135 million to $145 million.
- 2025 Research and Development Expenses Guidance: Expected to be in the range of $100 million to $105 million.
- 2025 G&A Expenses Guidance: Expected to be in the range of $35 million to $40 million.
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- Lexicon Pharmaceuticals Inc (LXRX, Financial) reported a significant increase in cash equivalents and short-term investments, ending 2024 with $238 million compared to $170 million in 2023.
- The company achieved early completion of enrollment for the Progress Phase 2B study of Pelliappodin, showing promising results in diabetic peripheral neuropathic pain (DPNP).
- Lexicon Pharmaceuticals Inc (LXRX) made substantial progress in the pivotal Phase 3 Sonata HCM study of cytogaflozin for hypertrophic cardiomyopathy (HCM), with patient enrollment proceeding as planned.
- The company entered a significant licensing agreement with Viris for the commercialization of Sotolozen outside the US and Europe, enhancing its business development efforts.
- Lexicon Pharmaceuticals Inc (LXRX) demonstrated strong financial performance with a reported revenue of $26.6 million in the fourth quarter and $31.1 million for the full year 2024, including a $25 million upfront payment from the Vatrice licensing agreement.
Negative Points
- Research and development expenses increased significantly to $26.7 million in the fourth quarter of 2024 from $14.8 million in the same period of 2023, reflecting higher investments in clinical trials.
- The company reported a net loss of $33.8 million for the fourth quarter of 2024, although this was an improvement from the $49.8 million loss in the corresponding period of 2023.
- Lexicon Pharmaceuticals Inc (LXRX) ceased all promotional efforts for MEPA in the US due to a challenging market access environment dominated by major SGLT2 inhibitors.
- Selling, general, and administrative expenses increased to $143.1 million for the full year 2024, up from $114 million in 2023, driven by higher marketing costs and employee-related expenses.
- The company faces potential challenges in the commercialization of its therapies, particularly in the US market, where it plans to navigate a different payer situation for HCM compared to heart failure.
