GT Biopharma, trading under the ticker GTBP, has successfully completed the dosing of Cohort 1 in its Phase 1 dose escalation trial for GTB-3650. This study is focused on treating relapsed or refractory hematologic malignancies that express CD33. Following a thorough safety evaluation, the company found no issues with safety or tolerability, allowing them to proceed with dosing in Cohort 2, which has now commenced.
The trial aims to assess GTB-3650's effectiveness in engaging and expanding natural killer (NK) cells within patients. Initial results from Cohort 1 indicate promising immunologic activity, suggesting the drug’s potential to activate endogenous NK cells. GT Biopharma intends to disclose more detailed findings in 2025 once further cohorts are completed and additional patients enrolled.
This trial will involve approximately 14 patients, with GTB-3650 administered in two-week treatment cycles for up to four months, depending on clinical outcomes. The research will investigate various parameters such as safety, pharmacokinetics, pharmacodynamics, and the in vivo expansion of patient NK cells.
Wall Street Analysts Forecast
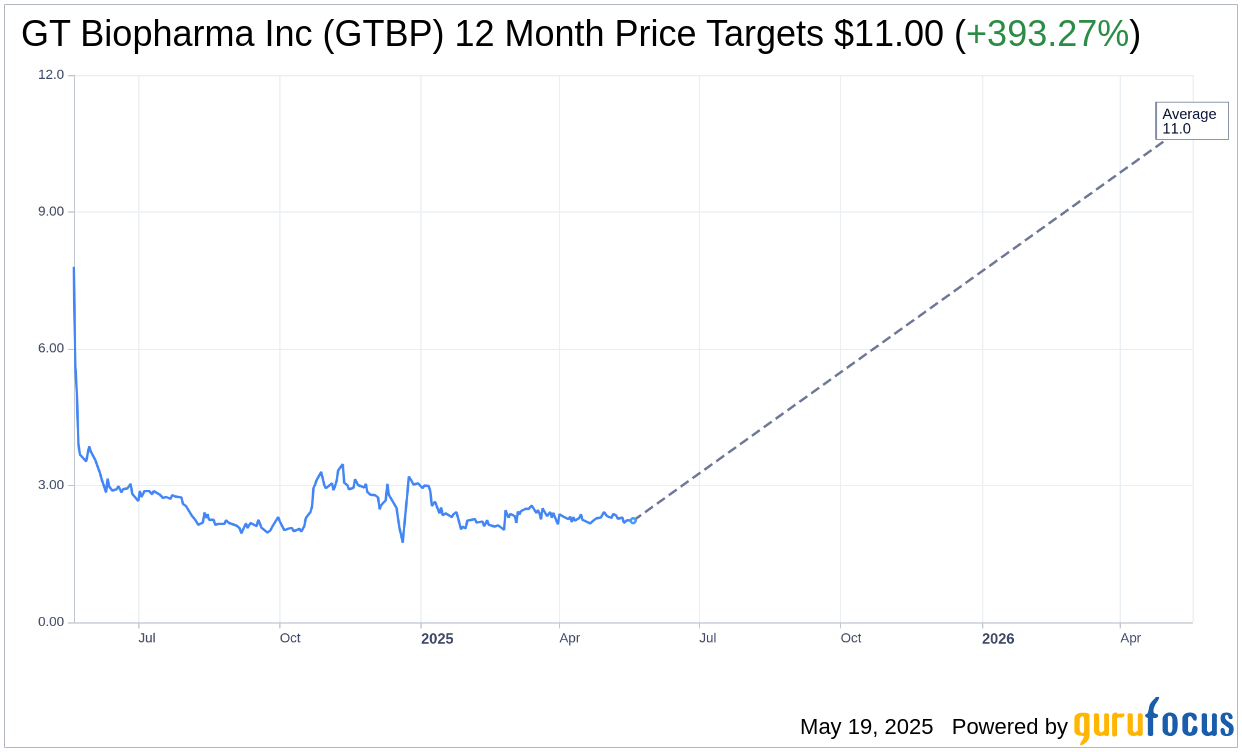
Based on the one-year price targets offered by 1 analysts, the average target price for GT Biopharma Inc (GTBP, Financial) is $11.00 with a high estimate of $11.00 and a low estimate of $11.00. The average target implies an upside of 393.27% from the current price of $2.23. More detailed estimate data can be found on the GT Biopharma Inc (GTBP) Forecast page.
Based on the consensus recommendation from 1 brokerage firms, GT Biopharma Inc's (GTBP, Financial) average brokerage recommendation is currently 2.0, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
