On May 19, Oncocyte (OCX, Financial) shared encouraging news about the updated pricing for its advanced lab-developed test, GraftAssureCore. The Centers for Medicare & Medicaid Services have increased the reimbursement rate to $2,753 per test outcome. This marks an improvement over the previous payment structure for an earlier version of the test, which reimbursed $2,222 for initial patient tests and $1,029 for follow-up tests. GraftAssureCore is conducted in Oncocyte's CLIA-certified laboratory located in Nashville.
Over the last two years, Oncocyte has significantly invested in enhancing the scalability and production efficiency of its testing processes to back its kit-based testing program. Last fall, the company integrated these advancements into its CLIA lab and submitted them for repricing to MolDX3. CEO Josh Riggs expressed satisfaction with the resulting repricing.
Wall Street Analysts Forecast
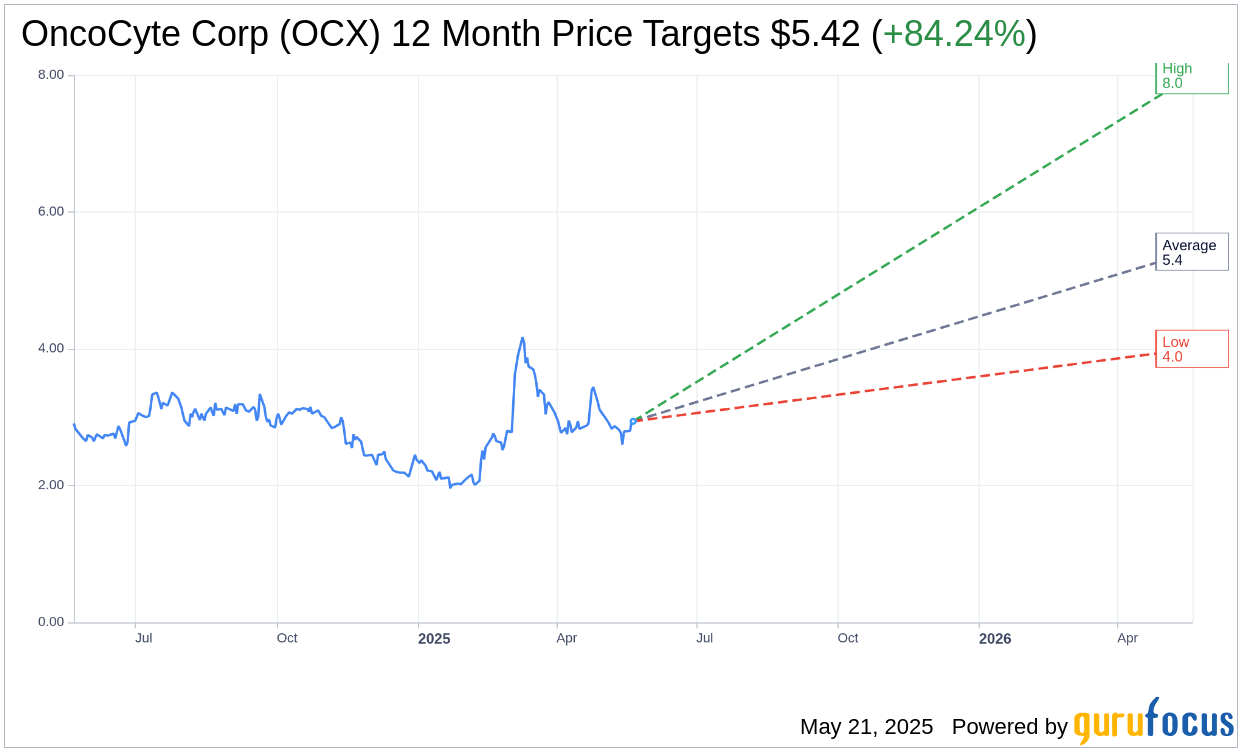
Based on the one-year price targets offered by 3 analysts, the average target price for OncoCyte Corp (OCX, Financial) is $5.42 with a high estimate of $8.00 and a low estimate of $4.00. The average target implies an upside of 84.24% from the current price of $2.94. More detailed estimate data can be found on the OncoCyte Corp (OCX) Forecast page.
Based on the consensus recommendation from 4 brokerage firms, OncoCyte Corp's (OCX, Financial) average brokerage recommendation is currently 2.5, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
OCX Key Business Developments
Release Date: May 12, 2025
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- OncoCyte Corp (OCX, Financial) has finalized its clinical trial design and received central IRB approval, marking a significant milestone in its strategic pivot towards transplant rejection testing.
- The company is on track to submit a data package to the FDA by the end of the year, with FDA approval targeted for the first half of 2026.
- There is strong interest from top transplant centers in the US and Germany to participate in the clinical trial, representing nearly 10% of US transplant volumes.
- OncoCyte Corp (OCX) has successfully expanded its RUO assay to 10 sites across the US, Germany, UK, Switzerland, Austria, and Southeast Asia, with researchers exploring new applications for the tests.
- The company reported Pharma Services revenue of $2.1 million, exceeding expectations and extending its cash runway, with gross margins of 62%.
Negative Points
- Pharma Services revenue is situation-driven and expected to vary, with Q2 revenue anticipated to be less than $500,000, highlighting potential revenue volatility.
- The company's oncology pipeline, while promising, is still in the early stages compared to its transplant focus, indicating a longer timeline for revenue generation in this area.
- OncoCyte Corp (OCX) is undergoing a corporate rename, which, although budget-conscious, may cause temporary brand recognition challenges.
- The company anticipates a couple of quarters with increased cash burn due to clinical trial costs and FDA-compliant software development, impacting financial stability.
- There is uncertainty regarding the speed at which transplant centers will adopt the new test post-FDA approval, as these centers are generally risk-averse and may require time to integrate new technologies.
