Keros Therapeutics (KROS, Financial) has released preliminary data from the TROPOS trial, a Phase 2 study aimed at assessing cibotercept combined with existing therapies for treating pulmonary arterial hypertension (PAH). Initially, on December 12, 2024, the company stopped the 3.0 mg/kg and 4.5 mg/kg treatment arms due to the detection of pericardial effusions at these dosages. Subsequently, on January 15, 2025, Keros decided to cease all dosing in the trial, including the 1.5 mg/kg and placebo groups, upon further reviews revealing additional cases of pericardial effusion.
Despite the premature conclusion of the trial, patient monitoring persisted through the end-of-trial visits. After a thorough examination of all safety and efficacy data, Keros Therapeutics has opted to terminate the development of cibotercept for PAH treatment. The company will now focus on evaluating potential development strategies for cibotercept in other medical areas, contingent upon the completion of a strategic alternative review process.
Wall Street Analysts Forecast
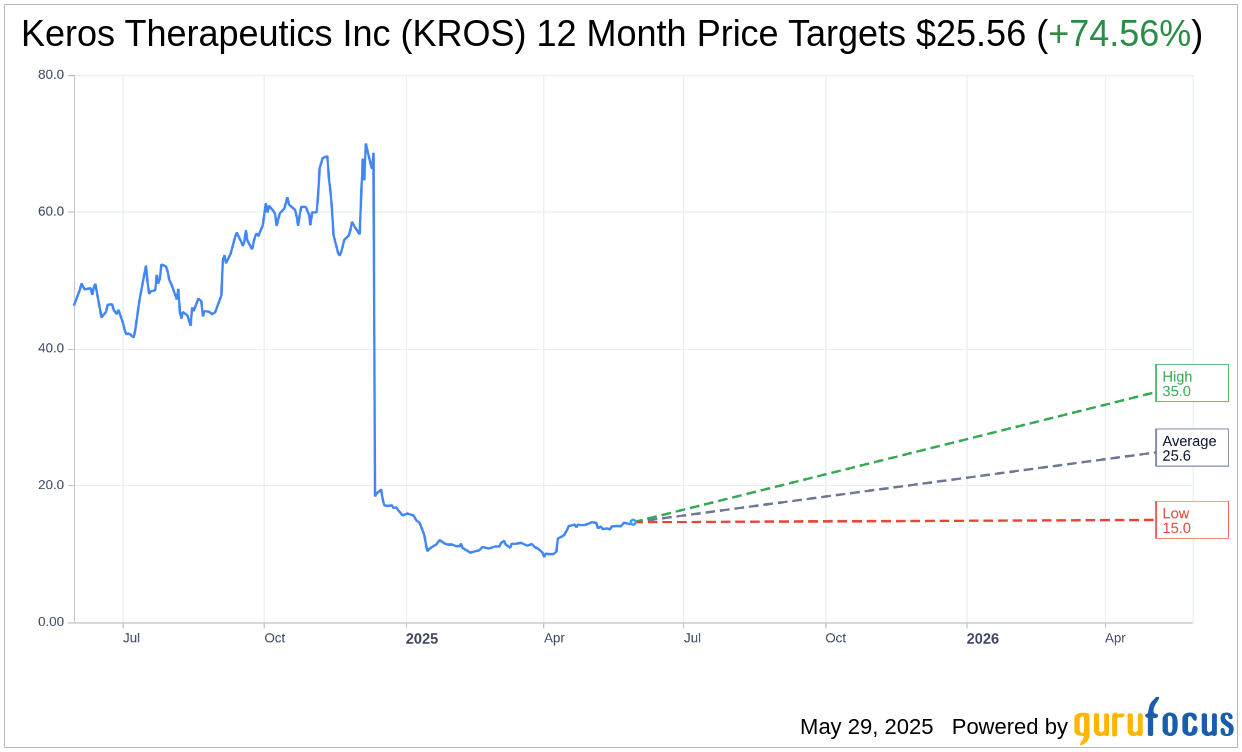
Based on the one-year price targets offered by 9 analysts, the average target price for Keros Therapeutics Inc (KROS, Financial) is $25.56 with a high estimate of $35.00 and a low estimate of $15.00. The average target implies an upside of 74.56% from the current price of $14.64. More detailed estimate data can be found on the Keros Therapeutics Inc (KROS) Forecast page.
Based on the consensus recommendation from 13 brokerage firms, Keros Therapeutics Inc's (KROS, Financial) average brokerage recommendation is currently 2.1, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
