Lipocine (LPCN, Financial) has taken a significant step forward with its licensing partner, Verity Pharma, by filing a New Drug Submission (NDS) for TLANDO in Canada. This development potentially opens the Canadian market to TLANDO, known for being the first and only oral testosterone replacement therapy approved by the U.S. Food and Drug Administration (FDA) that eliminates the need for dose adjustment.
The introduction of TLANDO in Canada is seen as an exciting opportunity. Lipocine's CEO, Mahesh Patel, highlighted this filing as a critical advancement towards offering a distinctive oral TRT solution in the region. The company anticipates that this innovation, once approved, could substantially enhance its market presence.
Wall Street Analysts Forecast
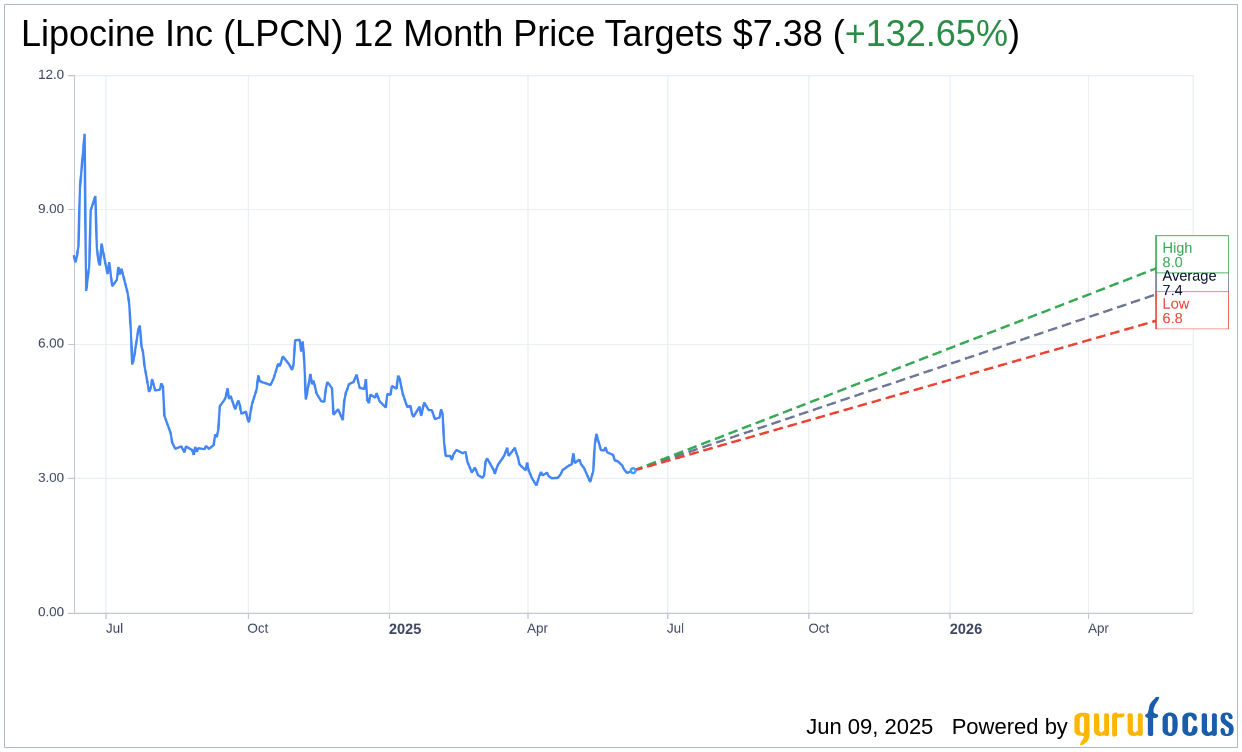
Based on the one-year price targets offered by 2 analysts, the average target price for Lipocine Inc (LPCN, Financial) is $7.38 with a high estimate of $8.00 and a low estimate of $6.75. The average target implies an upside of 132.65% from the current price of $3.17. More detailed estimate data can be found on the Lipocine Inc (LPCN) Forecast page.
Based on the consensus recommendation from 2 brokerage firms, Lipocine Inc's (LPCN, Financial) average brokerage recommendation is currently 2.0, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.