At a recent obesity-focused event organized by Evercore, key figures from Skye (SKYE, Financial) took part in a panel discussing advancements in non-incretin treatments. The team, including Punit Dhillon, Puneet Arora, MD, FACE, and Chris Twitty, PhD, shared insights alongside renowned obesity specialists. The panel examined the clinical and preclinical impacts of the CB1 pathway, emphasizing nimacimab's peripheral CB1 blockade, which provides an alternative approach to obesity management beyond the GLP-1 framework.
Chris Twitty, PhD, presented findings on nimacimab, spotlighting its significant weight loss effects in pre-clinical models of diet-induced obesity. The data suggests nimacimab's potential as a solo treatment or combined therapy. Additionally, new biomarker information revealed considerable reductions in obesity-related inflammation and liver steatosis.
Further, the event highlighted nimacimab's role in fostering metabolic balance, reflected in improved body composition and hormonal regulation. These studies support the peripheral CB1 inhibition strategy, using a monoclonal antibody that does not penetrate the blood-brain barrier, offering complementary benefits alongside incretin-based medications.
Wall Street Analysts Forecast
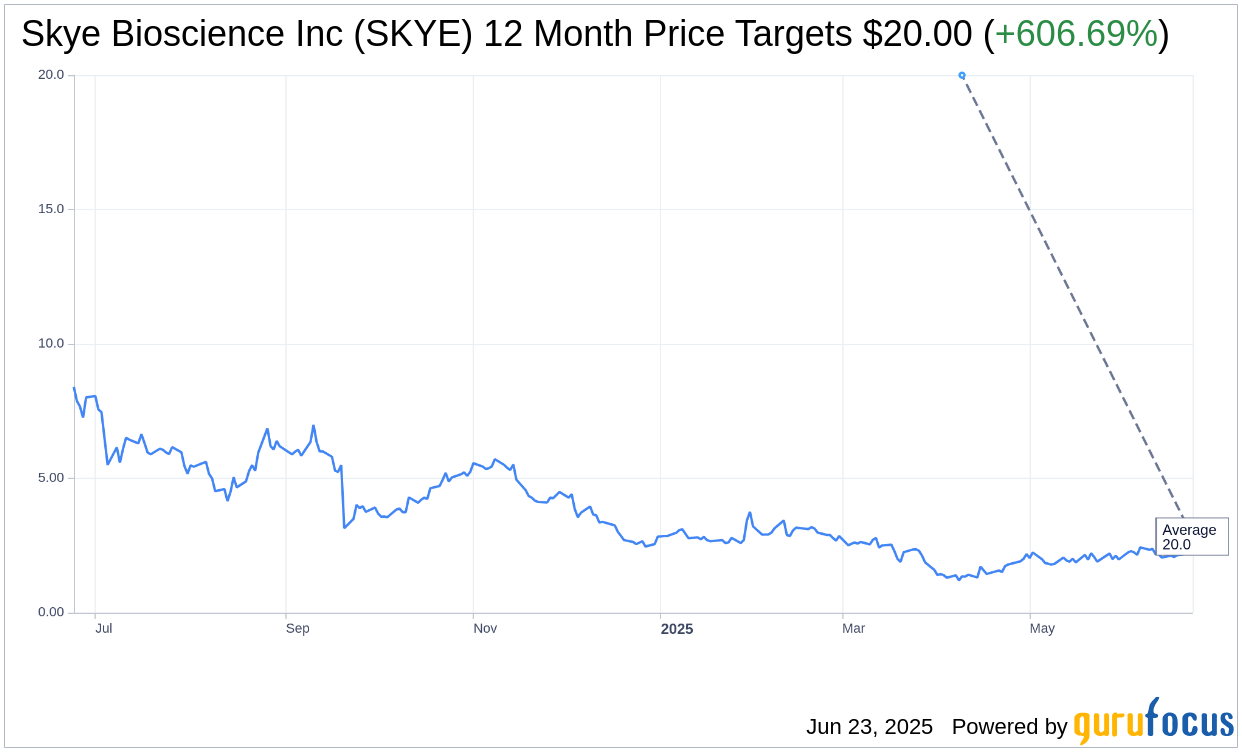
Based on the one-year price targets offered by 1 analysts, the average target price for Skye Bioscience Inc (SKYE, Financial) is $20.00 with a high estimate of $20.00 and a low estimate of $20.00. The average target implies an upside of 606.69% from the current price of $2.83. More detailed estimate data can be found on the Skye Bioscience Inc (SKYE) Forecast page.
Based on the consensus recommendation from 1 brokerage firms, Skye Bioscience Inc's (SKYE, Financial) average brokerage recommendation is currently 2.0, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
SKYE Key Business Developments
Release Date: May 08, 2025
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- Skye Bioscience Inc (SKYE, Financial) completed enrollment in their phase 2A CB1 trial ahead of schedule, allowing for an extension to 52 weeks to collect longer-term data.
- The company generated compelling new preclinical data validating the potential of nemasimab as a weight loss therapy, showing significant weight loss and glycemic control.
- Recent studies demonstrated that nemasimab can provide additive weight loss when combined with incretin mimetics like tirzepatide.
- The data safety monitoring committee completed three reviews with no safety concerns, indicating a strong safety profile for their ongoing trials.
- Skye Bioscience Inc (SKYE) has a solid financial position with cash and investments totaling $59.2 million, expected to fund operations through Q1 2027.
Negative Points
- The company faces regulatory uncertainty due to potential shifts in drug pricing policy and changes at the FDA and NIH.
- Research and development expenses increased significantly to $7.2 million from $1.9 million year-over-year, impacting financials.
- General administrative expenses also rose, primarily due to increased investor relations, marketing, and consulting costs.
- The net loss for the quarter was $11.1 million, more than double the loss from the same period in 2024.
- There is ongoing concern about the evolving policy environment and its potential impact on drug pricing and regulatory oversight.
