Goldman Sachs has initiated renewed coverage on Amylyx (AMLX, Financial), assigning a Buy rating with a target price of $10. This target suggests a potential increase of 58%. The investment firm holds a favorable perspective on avexitide, a treatment aimed at addressing hypoglycemia following bariatric surgery, viewing it as a potential blockbuster in the market.
Wall Street Analysts Forecast
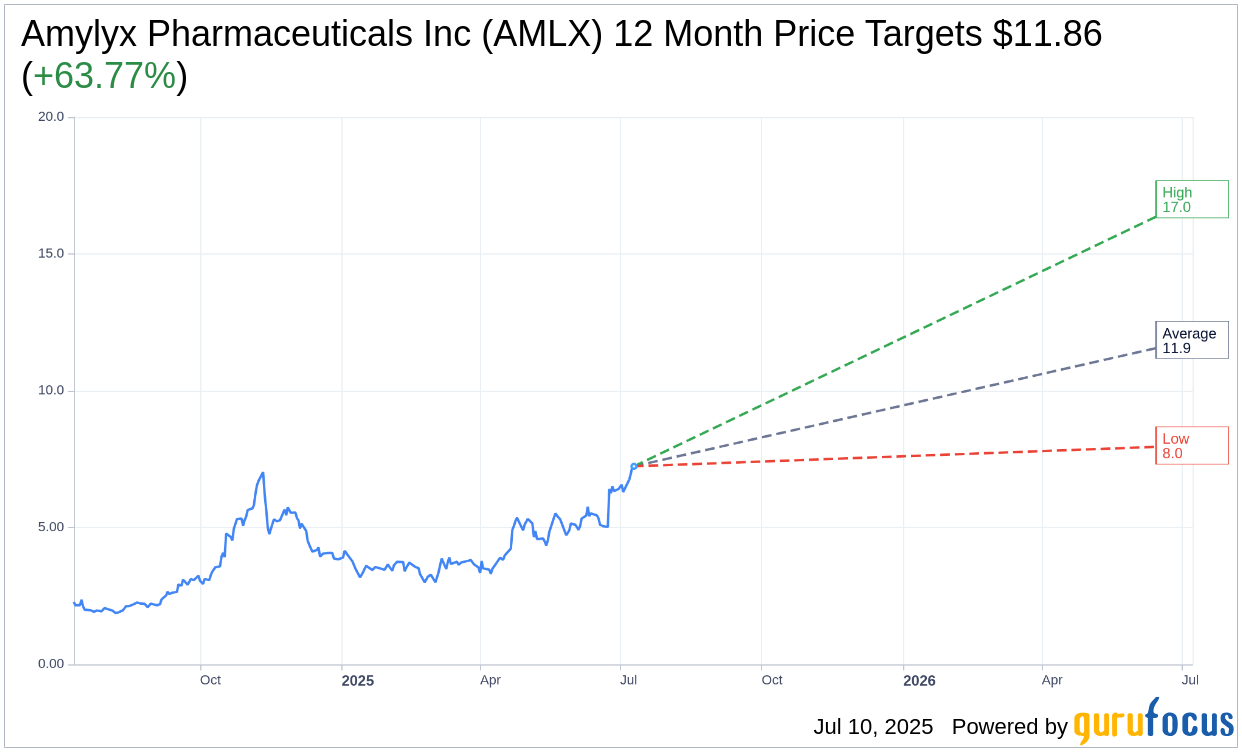
Based on the one-year price targets offered by 7 analysts, the average target price for Amylyx Pharmaceuticals Inc (AMLX, Financial) is $11.86 with a high estimate of $17.00 and a low estimate of $8.00. The average target implies an upside of 63.77% from the current price of $7.24. More detailed estimate data can be found on the Amylyx Pharmaceuticals Inc (AMLX) Forecast page.
Based on the consensus recommendation from 9 brokerage firms, Amylyx Pharmaceuticals Inc's (AMLX, Financial) average brokerage recommendation is currently 1.6, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
AMLX Key Business Developments
Release Date: May 08, 2025
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- Amylyx Pharmaceuticals Inc (AMLX, Financial) has achieved several meaningful milestones, including dosing the first participant in their pivotal phase 3 lucidity clinical trial for vexatide.
- The company has strengthened its financial position by raising approximately $65.5 million, extending their cash runway through the end of 2026.
- Positive topline data from the phase 2 Helios trial in Wolfram syndrome showed improvement or stabilization across all measured outcomes.
- The company is preparing for a potential commercial launch of vexatide in 2027, pending approval.
- Amylyx Pharmaceuticals Inc (AMLX) has a robust pipeline with multiple clinical trials targeting diseases with high unmet needs, including ALS and progressive supranuclear palsy.
Negative Points
- There are no currently approved treatments for post-bariatric hypoglycemia (PBH), which vexatide aims to address, indicating a high-risk development area.
- The company faces challenges in educating the medical community and patients about PBH and the potential benefits of vexatide.
- The phase 3 lucidity trial for vexatide is still in early stages, with topline data not expected until the first half of 2026.
- Amylyx Pharmaceuticals Inc (AMLX) has experienced a significant decrease in operating expenses, which may indicate reduced activity or cost-cutting measures.
- The company is dealing with residual cash obligations related to the discontinuation of previous products, which could impact financial flexibility.