Scotiabank has begun coverage of Gossamer Bio (GOSS, Financial), assigning it an Outperform rating and setting a price target of $11. The financial institution is optimistic about the company’s innovative strategies in addressing pulmonary arterial hypertension and pulmonary hypertension linked with interstitial lung diseases. Gossamer Bio’s primary drug candidate, seralutinib, is highlighted for its potential to gain traction due to its unique action as an inhaled tyrosine kinase inhibitor. This therapy is positioned as a complementary treatment alongside existing standard care, according to Scotiabank's analysis. The firm believes this could lead to significant advancement in the treatment landscape for these conditions.
Wall Street Analysts Forecast
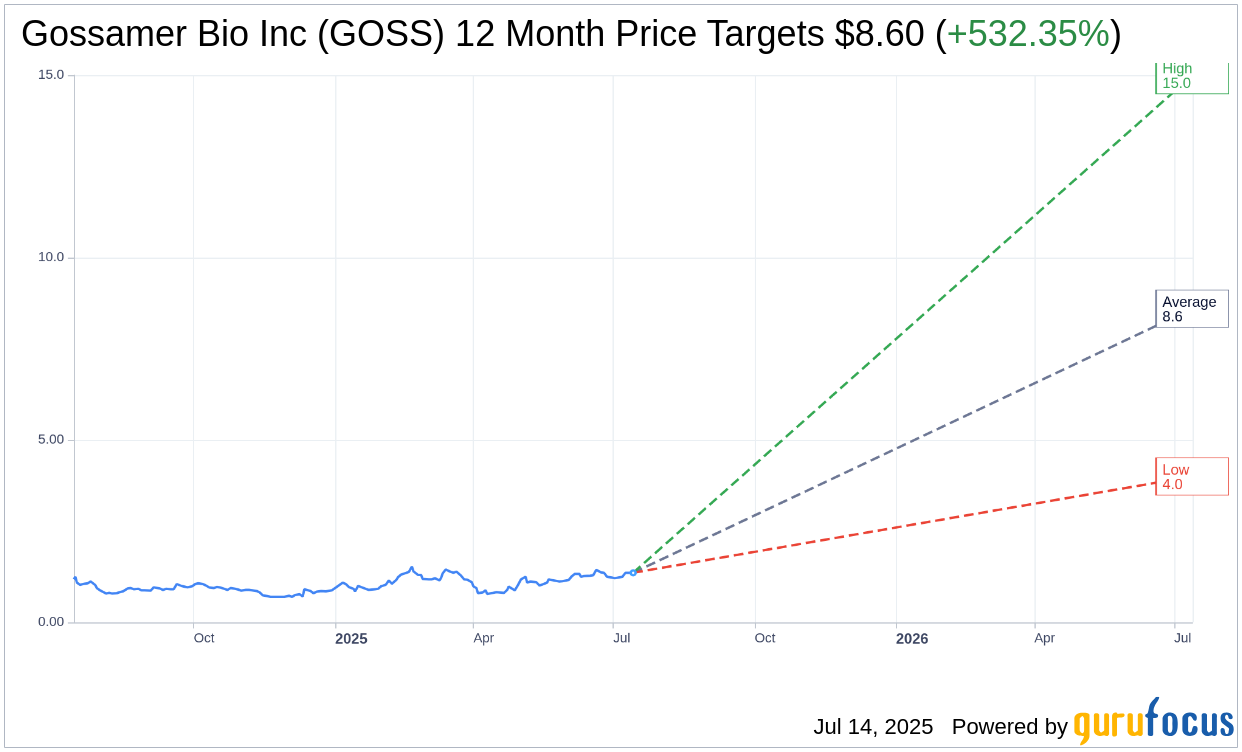
Based on the one-year price targets offered by 5 analysts, the average target price for Gossamer Bio Inc (GOSS, Financial) is $8.60 with a high estimate of $15.00 and a low estimate of $4.00. The average target implies an upside of 532.35% from the current price of $1.36. More detailed estimate data can be found on the Gossamer Bio Inc (GOSS) Forecast page.
Based on the consensus recommendation from 9 brokerage firms, Gossamer Bio Inc's (GOSS, Financial) average brokerage recommendation is currently 1.9, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
GOSS Key Business Developments
Release Date: May 15, 2025
- Cash and Cash Equivalents: $257.9 million at the end of Q1 2025.
- Revenue: $9.9 million for Q1 2025.
- R&D Expenses: $38 million for Q1 2025, up from $32.4 million in Q1 2024.
- G&A Expenses: $8.7 million for Q1 2025, down from $9.6 million in Q1 2024.
- Net Loss: $36.6 million or $0.16 per share for Q1 2025, compared to $41.9 million or $0.19 per share in Q1 2024.
- Collaboration Revenue: $6.6 million in cost reimbursements from Chiesi for Q1 2025.
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- Gossamer Bio Inc (GOSS, Financial) has achieved a significant milestone in the PROSERA study by closing new patient screening, indicating strong progress in their Phase 3 trial for seralutinib.
- The baseline characteristics of patients enrolled in the PROSERA study are precisely what Gossamer Bio Inc (GOSS) targeted, increasing optimism for achieving positive results.
- The company has a robust balance sheet with $257.9 million in cash and cash equivalents, providing sufficient capital until the first half of 2027.
- Gossamer Bio Inc (GOSS) has a strong partnership with the Chiesi Group, enabling seralutinib to enter a global Phase 3 study in PH-ILD, highlighting strategic collaborations.
- The company is targeting a substantial unmet need in PAH and PH-ILD, with potential for seralutinib to become a first-in-class treatment, addressing underlying disease mechanisms.
Negative Points
- The timeline for announcing top-line results from the PROSERA study has been pushed to February 2026, delaying potential market entry.
- Gossamer Bio Inc (GOSS) reported a net loss of $36.6 million for the first quarter of 2025, indicating ongoing financial challenges.
- Enrollment in the PROSERA study took longer than expected due to stringent criteria, which may have impacted timelines and resource allocation.
- The company faces competition from existing treatments like sotatercepts, which may influence market dynamics and patient enrollment.
- There is uncertainty regarding the regulatory approval process, as the FDA's specific requirements for 6-Minute Walk improvement are not clearly defined.
