- Aldeyra Therapeutics (ALDX, Financial) is aiming for a crucial FDA approval with its recent NDA resubmission.
- Analysts' price targets suggest a potential upside of nearly 68% for investors.
- The consensus on Wall Street leans towards a "Buy" recommendation for ALDX.
Aldeyra Therapeutics (ALDX) is making a strategic move to secure approval for its eye treatment as it resubmits the New Drug Application for reproxalap. This follows the FDA's earlier request for additional efficacy data to support its use in treating dry eye disease. The FDA has slated December 16, 2025, as the target date for its comprehensive review. Investors are closely watching these developments, as FDA approval could significantly impact Aldeyra's market standing and financial performance.
Wall Street Analysts Forecast
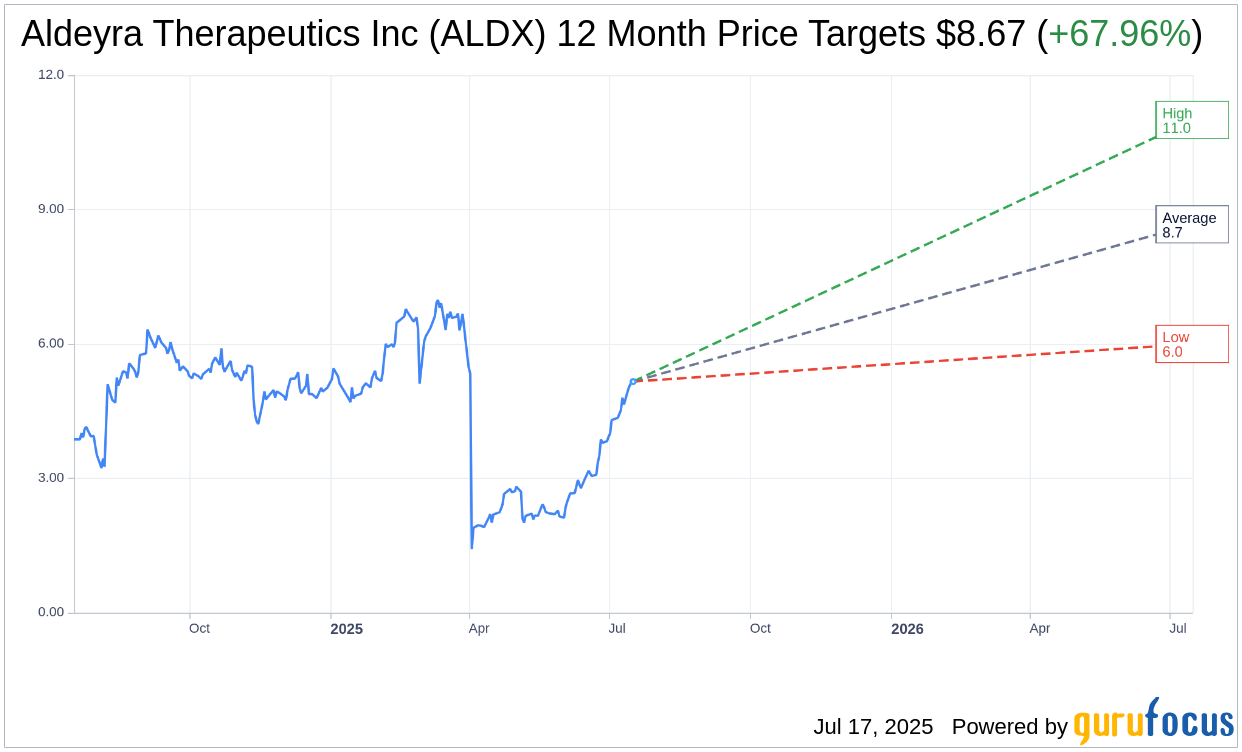
Analysts have provided one-year price targets for Aldeyra Therapeutics Inc (ALDX, Financial), forecasting an average target price of $8.67. This estimate includes a high target of $11.00 and a lower boundary of $6.00. Compared to the current trading price of $5.16, the average target indicates a potential upside of 67.96%. For further insights, visit the Aldeyra Therapeutics Inc (ALDX) Forecast page for detailed price projections.
Analysts' Recommendations
Currently, the consensus among six brokerage firms places Aldeyra Therapeutics Inc's (ALDX, Financial) average brokerage recommendation at 1.5. This suggests a "Buy" stance on the stock. The recommendation scale ranges from 1 to 5, with 1 representing a Strong Buy and 5 indicating a Sell. This optimistic outlook aligns with the promising potential seen in Aldeyra's pipeline and market strategy.
Investors considering ALDX should weigh the favorable analyst sentiment and the pending FDA decision, which could be pivotal for the company's future prospects.
