Edwards Lifesciences (EW, Financial) has announced the filing of an automatic mixed securities shelf. This move is designed to streamline the process of offering various types of securities in the future. By employing this strategy, the company aims to enhance its financial flexibility and capitalize on potential investment opportunities as they arise. The filing does not specify a particular amount or type of securities, allowing Edwards Lifesciences to respond swiftly to market conditions and fundraising needs. Investors will be watching closely to see how this strategy impacts the company's growth and development plans.
Wall Street Analysts Forecast
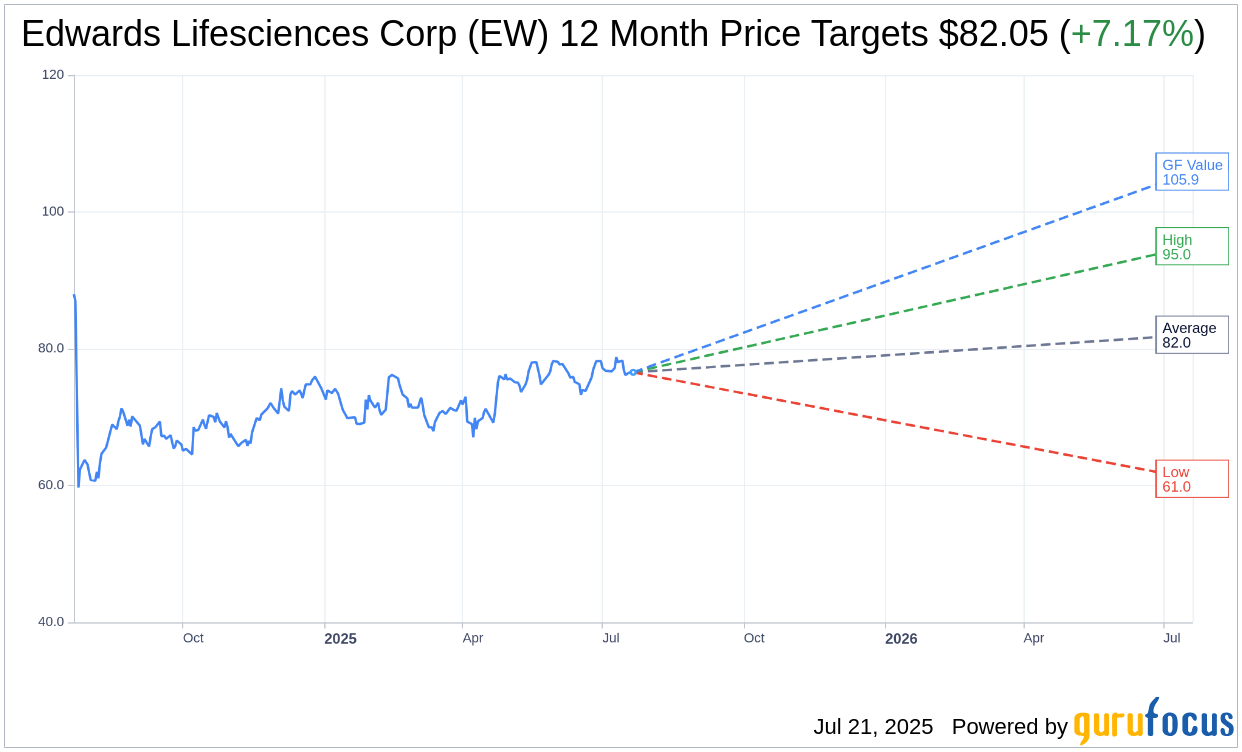
Based on the one-year price targets offered by 27 analysts, the average target price for Edwards Lifesciences Corp (EW, Financial) is $82.05 with a high estimate of $95.00 and a low estimate of $61.00. The average target implies an upside of 7.17% from the current price of $76.56. More detailed estimate data can be found on the Edwards Lifesciences Corp (EW) Forecast page.
Based on the consensus recommendation from 34 brokerage firms, Edwards Lifesciences Corp's (EW, Financial) average brokerage recommendation is currently 2.4, indicating "Outperform" status. The rating scale ranges from 1 to 5, where 1 signifies Strong Buy, and 5 denotes Sell.
Based on GuruFocus estimates, the estimated GF Value for Edwards Lifesciences Corp (EW, Financial) in one year is $105.91, suggesting a upside of 38.34% from the current price of $76.56. GF Value is GuruFocus' estimate of the fair value that the stock should be traded at. It is calculated based on the historical multiples the stock has traded at previously, as well as past business growth and the future estimates of the business' performance. More detailed data can be found on the Edwards Lifesciences Corp (EW) Summary page.
EW Key Business Developments
Release Date: April 23, 2025
- Total Company Sales: $1.41 billion, an 8% increase in Q1 2025.
- TAVR Sales: $1.05 billion, a 5.4% increase over the prior year.
- TMTT Sales: $115 million, representing about 60% growth.
- Surgical Sales: $251 million, a 3% increase over the prior year.
- Adjusted EPS: $0.64 for the quarter.
- GAAP EPS: $0.62 for the quarter.
- Adjusted Gross Profit Margin: 78.7% in Q1 2025.
- SG&A Expenses: $466 million, 33% of sales.
- R&D Expenses: $255 million, 18% of sales.
- Adjusted Operating Profit Margin: 29.1% for the quarter.
- Cash and Cash Equivalents: Approximately $3 billion at the end of the quarter.
- Share Repurchase: $300 million repurchased in Q1 2025.
- Full Year Sales Guidance: Increased to $5.7 billion to $6.1 billion.
- Full Year EPS Guidance: $2.40 to $2.50.
- Q2 2025 Sales Guidance: $1.45 billion to $1.53 billion.
- Q2 2025 EPS Guidance: $0.59 to $0.65.
For the complete transcript of the earnings call, please refer to the full earnings call transcript.
Positive Points
- Edwards Lifesciences Corp (EW, Financial) reported an 8% increase in total company sales, reaching $1.41 billion in the first quarter of 2025.
- The company raised its 2025 TMTT sales guidance range to $530 million to $550 million, reflecting strong momentum in its transcatheter mitral and tricuspid therapies.
- Edwards Lifesciences Corp (EW) achieved significant milestones, including the approval of Sapien M3 in Europe, the world's first transcatheter mitral valve replacement system.
- The company maintained its full-year total company sales growth guidance of 8% to 10%, demonstrating confidence in its strategic plans.
- Edwards Lifesciences Corp (EW) has a strong balance sheet with approximately $3 billion in cash and cash equivalents, providing financial flexibility for future investments.
Negative Points
- The company faces potential impacts from tariffs and the JenaValve acquisition, which could affect future financial results.
- Edwards Lifesciences Corp (EW) experienced weaker procedure growth and competitive pressure in Japan, impacting sales performance in the region.
- The company anticipates pressure on its operating margin due to the weakening dollar and announced tariffs.
- There is uncertainty regarding the timing of the national coverage decision (NCD) for TAVR, which could impact the expansion of treatment centers.
- The launch of the Sapien M3 mitral valve replacement system in Europe is expected to be gradual, with a focus on creating a new category and achieving excellent patient outcomes.
